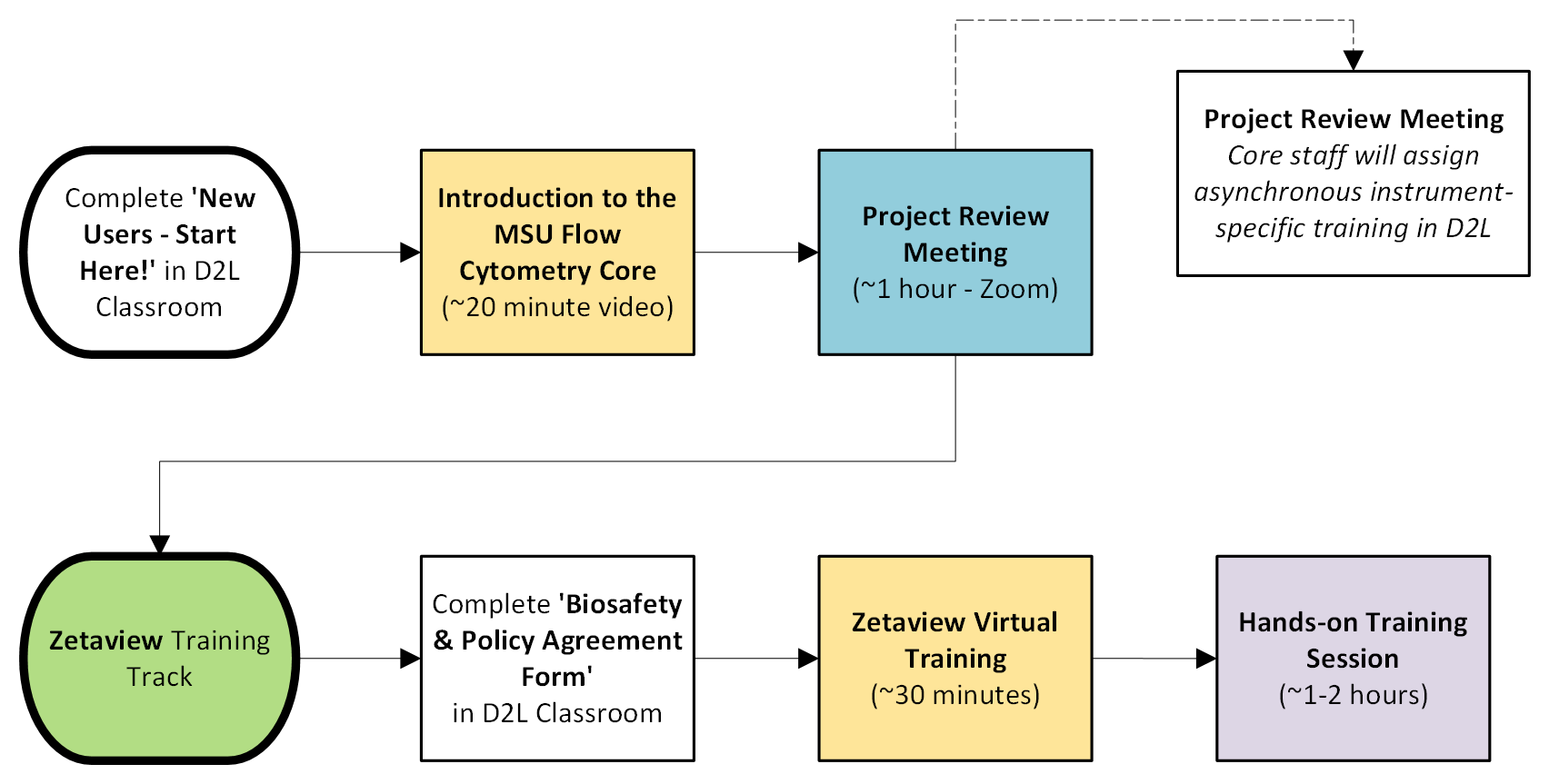
New Users
All individuals interested in independently operating benchtop analyzers or utilizing cell sorting services within the Core, must complete basic training within our D2L Training Community.
If you are interested in using Core services or receiving updates from the Core Facility, please click on the link below or contact us at FACS@msu.edu in order to be added to the MSU Flow Cytometry Core D2L Training Community or Core email listserv.
If you are interested in scheduling an initial consultation with Core Staff, please use the link below to arrange a time to meet.
Training Track Overview
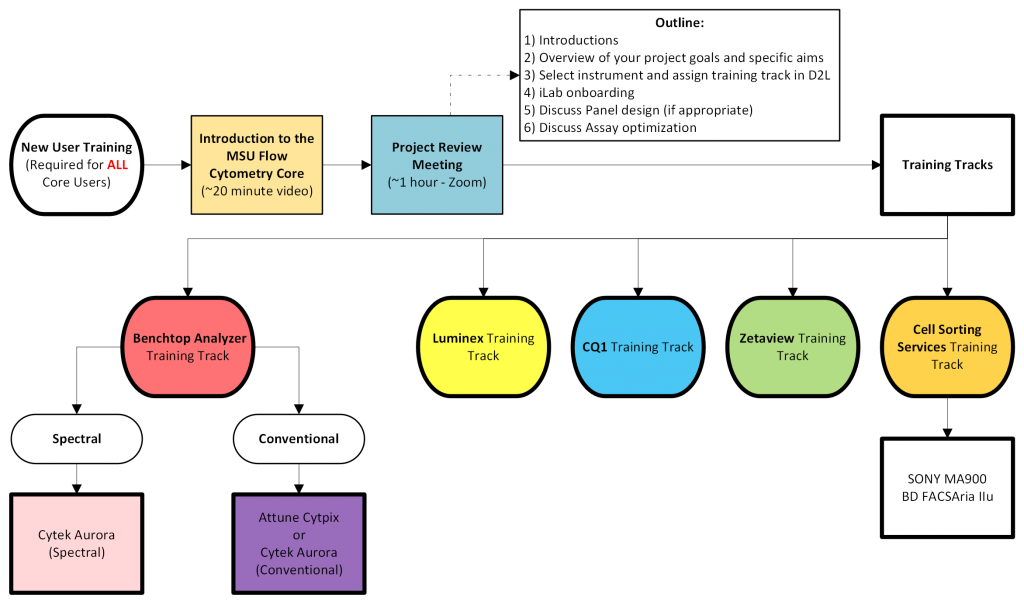
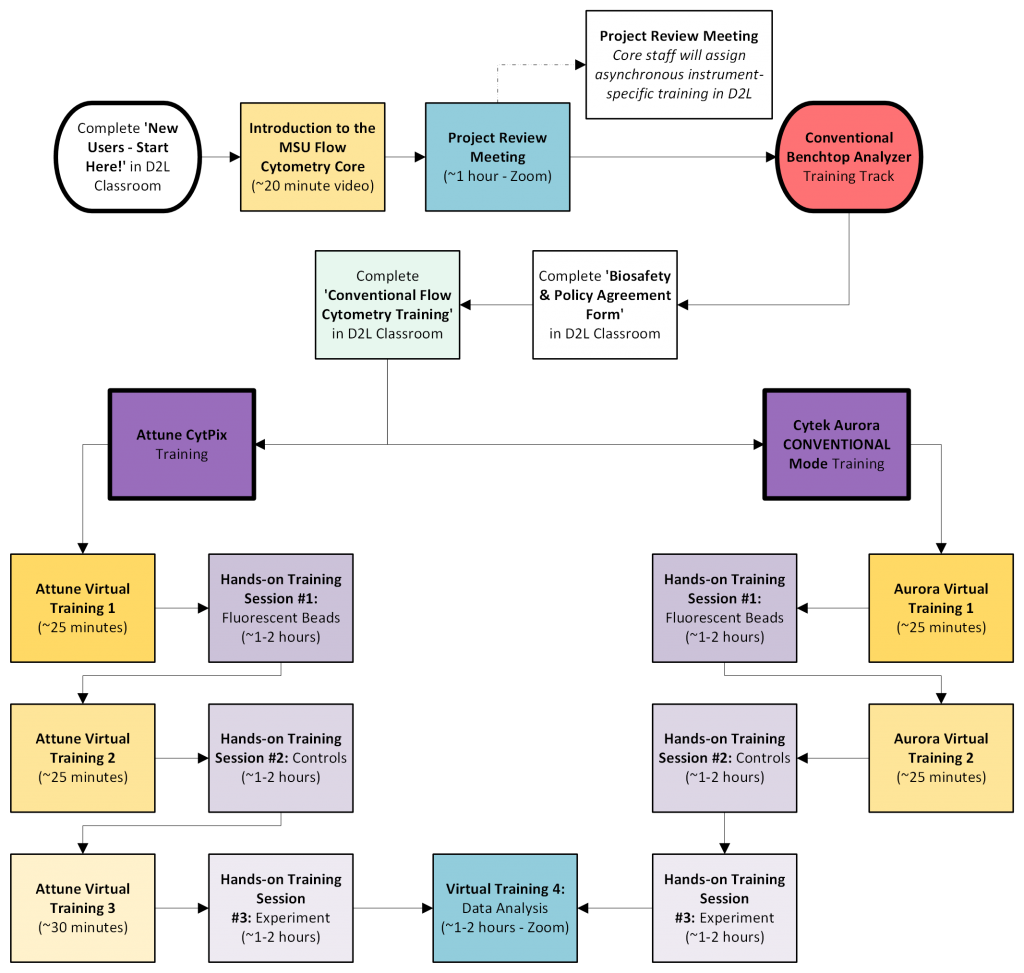
Conventional Benchtop Analyzer Training Track
This Training Track is relevant for receiving training on the Attune CytPix or Cytek Aurora. If your experiments are time sensitive and need to be analyzed before you can be trained to use the instruments independently, email FACS@msu.edu to arrange time for Core Staff to run your samples.
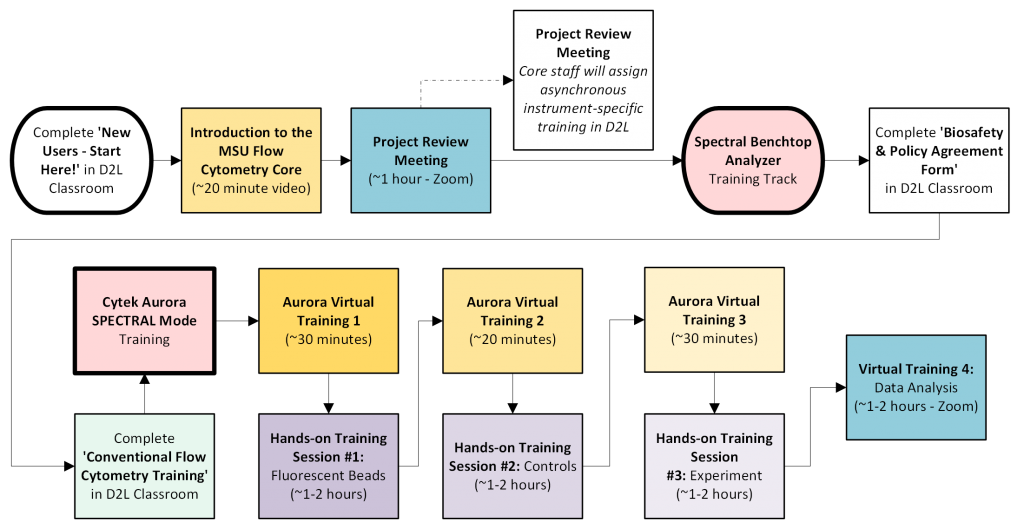
Spectral Benchtop Analyzer Training Track
This Training Track is relevant for receiving spectral cytometry training on the Cytek Aurora. If your experiments are time sensitive and need to be analyzed before you can be trained to use the instruments independently, email FACS@msu.edu to arrange time for Core Staff to run your samples.
Cell Sorting Services Training Track
This Training Track is relevant for utilizing cell sorting services on the SONY MA900 or the BD FACSAria IIu.
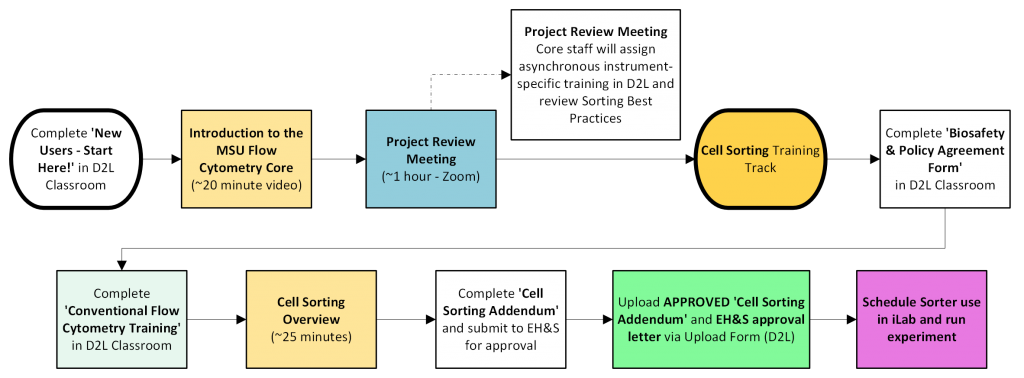
- All Users must complete a MSU Flow Cytometry Core Cell Sorting Addendum: All materials to be sorted must be approved in your lab’s Safety Protocol within HURON Click. An addendum for projects involving sorting on a flow cytometer must be completed and approved by EH&S and/or the IBC. This addendum is required due to the increased risk of aerosolization when sorting using an electrostatic-based cell sorter. Our policies are set based upon NIH Sorter Biosafety Guidelines and ISAC (International Society for Advancement of Cytometry) Sorter Biosafety Standards. The Cell Sorting Addendum should be uploaded as an amendment to your lab’s existing HURON Click Safety Protocol. If you are a student, postdoc, or staff member, please let your PI know that this submission and approval in the HURON Click system is required prior to any approval of cell sorting by the Core.
Review our Sorting Best Practices, which outlines important information regarding appropriate sample buffer, collection buffers, filtering samples, sample concentrations, etc.
High Content Imaging (CQ1) Training Track

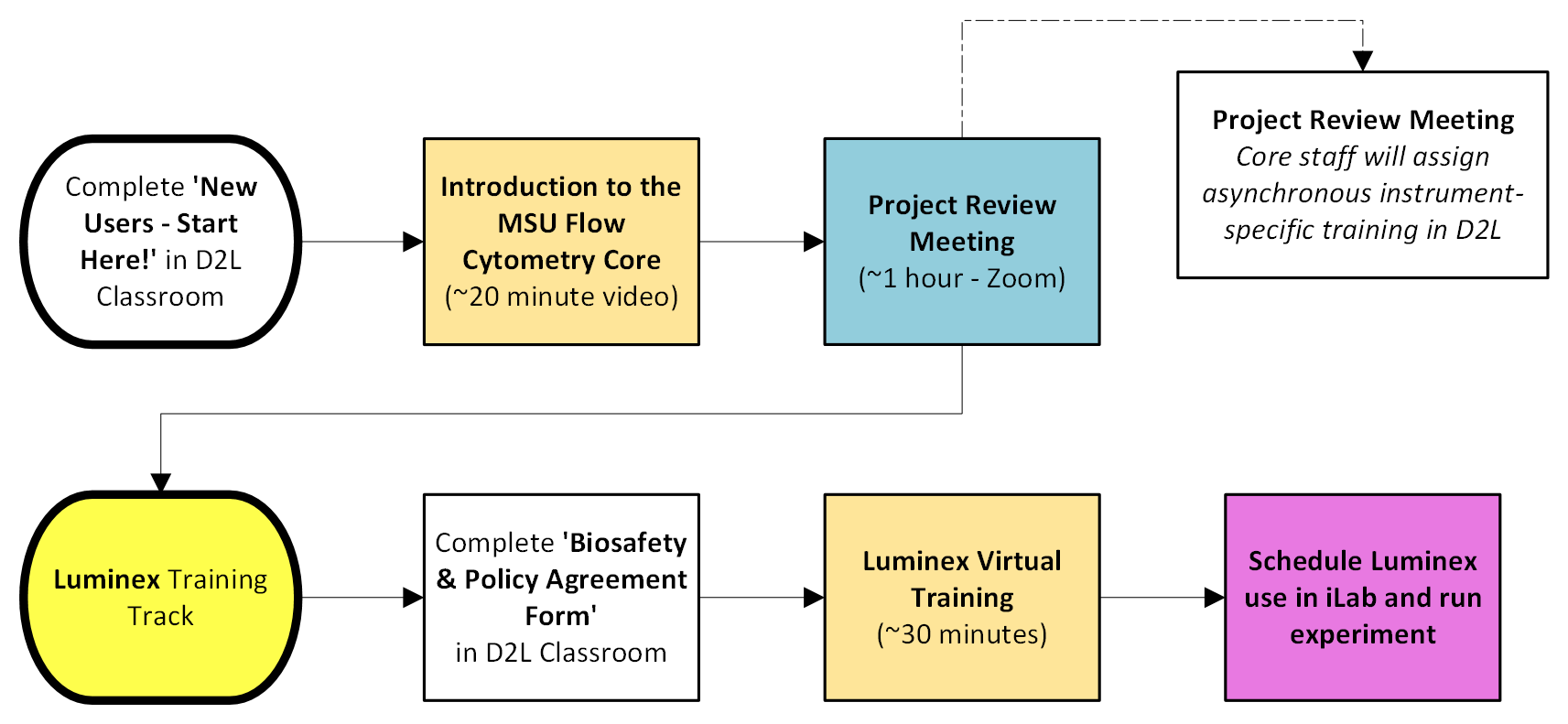
Luminex 200 Training Track
ZetaView Training Track